Safety & Security
Vacuum Systems Protection
Vacuum lines are used commonly in research labs. Proper set-up and maintenance of laboratory vacuum lines are important to protect the central vacuum system as well as facility maintenance staff who service this equipment. The vacuum system should be protected by a HEPA filter, particularly when used to manipulate biohazardous materials/infectious waste, and an overflow container should be present and managed vigilantly. Lab materials should never reach the main vacuum pump and potentially expose maintenance staff to biohazardous/chemical agents.
According to the Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition, in Biosafety Containment Level 2 (BSL-2) laboratories vacuum lines should be protected with liquid disinfectant traps. Additionally, if vacuum lines are used with toxins, they should be protected with a HEPA filter.
filters and flasks
The aspiration of tissue culture media from monolayer cultures and of supernatants from centrifuged samples into primary collection flasks is a common laboratory procedure.
- A HEPA filter provides an effective barrier to protect the vacuum system.
- Flexible tubing is used of appropriate inside diameter for the flask and filter fittings
and of sufficient wall thickness for the applied vacuum. Double check that all seals
and connections are secure.
- Flasks should be of appropriate size to contain the amount of fluid aspirated. Monitor
the levels in the flasks and do not allow them to overflow.
- Flasks should contain an appropriate disinfectant solution. The volume of disinfectant
should be such that the final concentration will be 10% (e.g. for a 500ml flask, one
should have 50ml of standard household bleach). Use an antifoam additive to prevent
foam production, if allowed to reach the filter, foam will shut off the vacuum.
- If the filter becomes contaminated or requires changing, the filter and flask can be safely removed by clamping the line between filter and vacuum source. Replace the HEPA filter when it becomes clogged or if it comes into contact with liquid.
According to the BMBL 5th Ed., aspirator bottles or suction flasks should be connected to an overflow collection flask containing appropriate disinfectant, and to an in-line HEPA or equivalent filter (Figure 1). This combination will provide protection to the central building vacuum system or vacuum pump, as well as to the personnel who service this equipment. Inactivation of aspirated materials can be accomplished by placing sufficient chemical decontamination solution inactivate the infectious waste as they are collected. Once inactivation occurs (contact time of at least 30min), liquid materials can be disposed of as noninfectious waste. Per the USF biomedical waste plan, (PDF) this solution can then be disposed of down the sanitary drain.
It is recommended that this setup be placed into a secondary container capable of holding the total volume of both flasks.
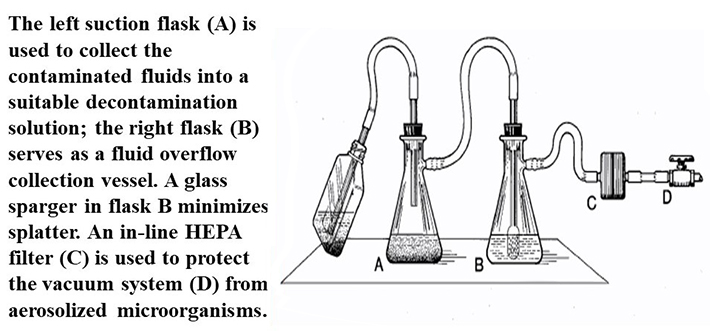
Figure 1 (U.S. Department of Health and Human Services Public Health Service Centers for Disease Control and Prevention National Institutes of Health HHS Publication No. (CDC) 21-1112 Revised December 2009). One method to protect a house vacuum system during aspiration of infectious fluids. The left suction flask (A) is used to collect the contaminated fluids into a suitable decontamination solution; the right flask (B) serves as a fluid overflow collection vessel. An in-line HEPA filter (C) is used to protect the vacuum system (D) from aerosolized microorganisms.
How to disinfect tissue culture media in vacuum flasks
- Prepare the primary vacuum flask with bleach.
Add bleach to the primary vacuum flask to equal 10% of the maximum collection volume. Maximum collection volume should be no more than two-thirds full. - Label the flask.
Label the flask as follows: "Tissue culture media disinfected with bleach 9:1"
- Protect the vacuum system with a filter.
Insert an in-line hydrophobic HEPA filter before the vacuum outlet. -
Add the tissue culture media to be disinfected.
-
Aspirate tissue culture media into bleach in the flask to make a final concentration of 10% bleach solution (9 parts tissue culture media: 1 part straight bleach).
-
After reaching maximum volume, allow a minimum 30-minute contact time.
-
Empty the flask into the laboratory sink.
-
For questions regarding the setup and use with biohazardous agents, contact Biosafety program.
Resources
CDC Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition