DEA-Controlled Substances and Research
DEA-Controlled Substances and Research
Use of Drug Enforcement Agency (DEA)-Controlled Substances in Research Settings
Introduction
Welcome to the resource page for the use of DEA-controlled substances in non-clinical research settings. This page aims to provide comprehensive information and support for researchers working with controlled substances to ensure compliance with federal regulations and promote safe and effective research practices.
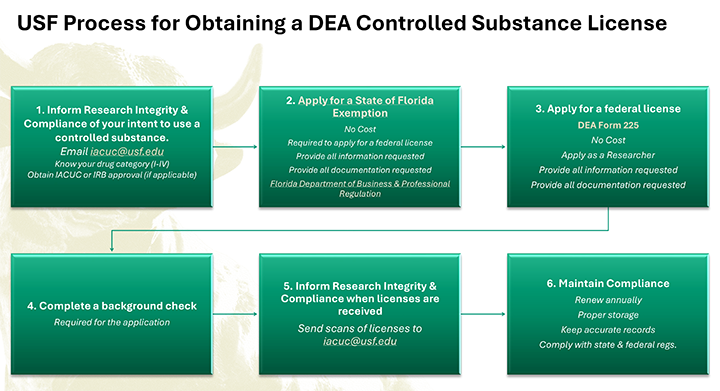
To conduct research at USF involving any DEA Controlled Substance in a non-clinical setting (preclinical studies/laboratory research with or without animals), Principal Investigators must obtain a DEA license.
Overview of DEA-Controlled Substances
The procurement, distribution, use, security, and record-keeping of controlled substances regulated by the DEA are described in 21 CFR 1300-1308.
Any faculty member requesting, possessing, or using any Schedule I controlled substance in preclinical studies/laboratory research with animals or Schedule II-V controlled substance in research without animals, should notify Research Integrity & Compliance (RIC) at RSCH Controlled Substances rsch-controlledsubstances@usf.edu.
DEA licensees are responsible for complying with all aspects of this policy. Registrants must identify the controlled substance(s) used in preclinical studies/laboratory research with or without animals, the individual(s) responsible for assisting in their compliance with these processes, and the location where the controlled substance will be securely stored, and they must ensure that complete records will be maintained. Faculty must ensure controlled substances are stored in an area of limited access and securely locked in a substantially constructed cabinet. Controlled substances must be secured behind two locks. Laboratory doors can be considered one lock; laboratory doors must be closed and locked when nobody is present.
Principal Investigators are responsible for maintaining accurate records of controlled substances in their possession by recording, on the Controlled Substance Record of Use Log, the amount used and the amount remaining in each vial.
Laboratories, storage cabinets, logs of use, and inventory records are subject to unannounced inspections and audits by the DEA and RIC.
Explanation of DEA Schedules
The Drug Enforcement Administration (DEA) classifies substances regulated under federal law into five distinct categories, or schedules, under the Controlled Substances Act (CSA).
Classification Considerations
The classification of substances into these schedules is based on several factors, including:
- Potential for Abuse: The likelihood that a substance will be misused or abused.
- Medical Use: Whether the substance has an accepted medical application in the United States (US).
- Safety and Dependence: The potential for a substance to cause psychological or physical dependence and the relative safety of its use.
Schedule I: No accepted medical use and high potential for abuse (e.g., heroin, LSD)
Schedule II-V: Accepted medical use with varying levels of potential for abuse (e.g., cocaine, methamphetamine, codeine)
Please check the resource DEA Researchers Manual 2022 (PDF) for details.
Who Needs to Apply?
If your research involves the use of any Schedule I-V controlled substance in preclinical studies in research labs, you must obtain appropriate approvals from the following.
- Federal Level: Drug Enforcement Administration (DEA)
- State Level: Florida Department of Health
- University Level: Institutional Animal Care and Use Committee (IACUC) as applicable
Regulatory Requirements
- Florida’s statutes regarding controlled substances are primarily found in Chapter 893 of the Florida Statutes, known as the Florida Comprehensive Drug Abuse Prevention and Control Act.
- The act classifies controlled substances into Schedules I through V, similar to federal classifications, with Schedule I substances having the highest potential for abuse and no accepted medical use.
Determine the Need and Requirements
- Identify Controlled Substances: Determine which controlled substances you need for your research and their respective schedules.
- Category I Substances: These are substances classified as having a high potential for abuse with no accepted medical use in the US (e.g., LSD, heroin).
- Ensure that your research is legitimate: The DEA is stringent with Category I substances.
- Understand Your Role: Identify whether you will be a researcher or in another role that requires DEA registration.
State Licensing Requirements
State Registration: Prior to requesting a DEA license, researchers must first obtain a controlled substances exemption from the Florida Department of Business and Professional Regulation (DBPR). This is required for anyone who intends to manufacture, distribute, or conduct research with controlled substances in Florida.
Application Process
Register with the State of Florida
Application: You need to apply to the DBPR. The specific form depends on whether you’re a practitioner, researcher, manufacturer, or distributor. For research, you would typically use Form DBPR-DDC-227 (PDF) or the online form.
- Obtain a Florida exemption status for non-clinical researchers.
- Background Check: A background check is often required, particularly when applying to handle Schedule I substances.
- Fee: USF researchers are exempt from the DBPR registration fees.
Register with the DEA
- You may file a DEA registration for controlled substances once a Florida DBPR exemption number is received. Registrations can be completed online using the 225 application. USF researchers are exempt from the DEA registration fees. Please enter the PI’s department chair or dean’s name under the “Official’s Name” on the application
- Submit Application: Submit the completed application form along with the required fee. Online and mail submissions are available. Make sure all sections are completed to avoid delays.
Site Inspection
The DEA may conduct a site inspection to ensure the security of the controlled substances. This includes:
- Proper storage facilities (e.g., safes, locked cabinets)
- Adequate record-keeping systems
- Procedures to prevent theft or diversion
Wait for DEA Approval
- The DEA will review your application and may request additional information.
- Approval time can vary, so it’s essential to apply well in advance of when you plan to start your research.
Post-Approval Compliance
After receiving the license, it is important to maintain strict compliance with DEA regulations as follows:
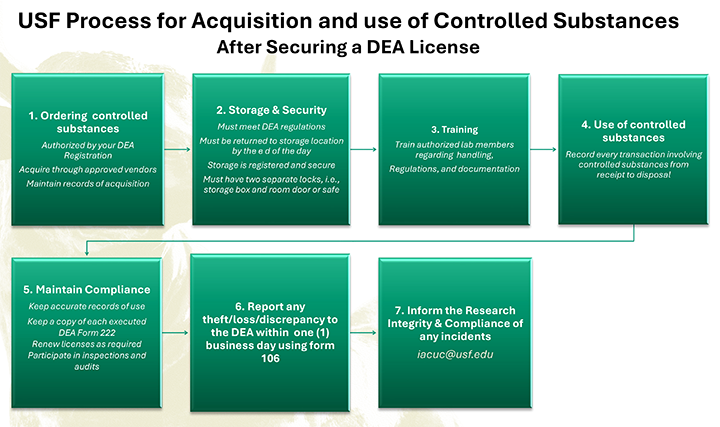
- Notify: Licensees must notify RIC and provide a record of their licenses as well as any controlled substances they will be storing/using.
- Inventory: Maintain accurate inventory records of the controlled substances.
- Security: Ensure that all controlled substances are stored securely.
- Record Keeping: Keep detailed records of all transactions involving controlled substances (e.g., usage, disposal).
Security and Record-Keeping
- Florida law requires that controlled substances be stored securely, similar to federal requirements.
- Accurate records must be kept regarding the acquisition, storage, use, and disposal of controlled substances.
Renewal
- DEA licenses are typically valid for one year and must be renewed annually.
- Researchers will qualify for an exemption from the licensing fee.
Penalties for Non-Compliance
Florida has strict penalties for non-compliance with controlled substances laws, including fines, loss of licensure, and potential criminal charges.
Support
Our assistance for controlled substances includes:
- Helping investigators identify requirements for the registration and use of controlled substances.
- Helping investigators maintain compliance with policies the University may issue for controlled substances.
- Monitoring and tracking investigators' Drug Enforcement Administration (DEA) registrations.
- Helping faculty and staff identify and dispose of substances and substances-resources.
- RIC does not obtain or maintain DEA licenses for investigators. Investigators are responsible for obtaining and maintaining licenses directly with the DEA.
Resources
- Florida Department of Business and Professional Regulation (DBPR): For more information on applications and forms
- Florida Statutes Chapter 893: To review the specific legal language